Sanitation surrounding the environment _ room milk reservoir includes sanitation air, floor, table, walls, and ceiling [1]. Disinfection process For sanitation room chemistry synthetic. Chemicals _ synthetic own excess that is can reduce bacteria quickly, but also lack that is formation residual and challenging to decompose [2]. As a result, it's necessary to utilize fewer synthetic chemicals and more natural substances.
Although maize is widely grown, very few people use silk, which they view as a waste [3]. Corn silk contains flavonoids and steroids or triterpenoids [4,5]. Chlorogenic acid, p-coumaric acid, ferulic acid, saponins, phytosterols, essential oils, resins, sugars, allantoin, and tannins are among the substances found in corn silk [6]. Minerals Ca, K, Mg, N, and Zn are present in corn silk. Corn silk also contains hormones, steroids, vitamins, and carbs [7,8].
Gram-positive bacteria like Staphylococcus aureus, Bacillus cereus, Bacillus subtilis, and Pseudomonas aeruginosa as well as gram-negative bacteria like Shigella sonnei, Shigella flexneri, Enterobacter aerogenes, Salmonella typhi, and Salmonella paratyphi can all be prevented from growing by using corn silk as an antibacterial. The components of corn silk have antimicrobial properties. For example, Escherichia coli and Staphylococcus aureus, two gram-negative and gram-positive bacteria, can both grow more slowly as a result [9].
Due to flavonoids' suppression of DNA and RNA production, sweet corn hair has a preference for the gram-positive bacteria it prefers to assault [10]. In order to prevent the growth of S. aureus and E. coli, the tannins included in sweet corn can enter bacterial cells that have been destroyed by saponins and flavonoids. For E. coli and S. aureus, the inhibitory zone diameters are 20 mm and 18 mm, respectively. In another study, sweet corn extract was tested at concentrations of 5, 10, and 15 mg/ml. The results showed that the concentrations of ethanol solvent with sweet corn extract at 10 and 15 mg/ml had a natural effect on preventing bacterial growth [11].
Comparatively to 65 and 75 percent ethanol, 70 percent ethanol solvent is the ideal concentration for dissolving flavonoid compounds [12]. With ethanol, which has a concentration above 70%, total flavonoids decrease [13]. According to research by Nurani et al. (2022) [14], the antibacterial activity of corn hair ethanol extract increased with increasing the concentration of the extract used. The concentrations tested were 10 %, 20 %, 30%, 40 %, 50%, 60 %, 70 %, 80 %, and 90 % with five repetitions each. A larger zone of bacterial inhibition width was visible in maize silk extracts with greater ethanol content. With concentrations >70%, the antibacterial activity of corn hair ethanol extract against S. aureus reduced.
This study aims to determine whether sweet corn hair extract can act as a natural disinfectant by lowering bacterial counts and bacterial inhibitory power in milk storage rooms. It also aims to determine the concentration of corn hair extract that has the greatest impact on lowering bacterial counts and bacterial inhibitory power in milk storage rooms.
Sanitation of the air, floor, table, walls, and ceiling is included in the sanitation of the environment room milk reservoir [1]. Synthetic sanitation room disinfection procedure. Chemicals are manmade and have an excess that can quickly eliminate microorganisms, but also a shortage that makes them difficult to breakdown [15]. As a result, it's necessary to utilize fewer synthetic chemicals and more natural substances.
Even though maize is commonly farmed, very few people use it since they consider it to be a waste [16]. Flavonoids, steroids, and triterpenoids are present in corn silk [4,5]. Chlorogenic acid, p-coumaric acid, ferulic acid, saponins, phytosterols, essential oils, resins, sugars, allantoin, and tannins are among the substances found in corn silk [6]. Minerals Ca, K, Mg, N, and Zn are present in corn silk. Corn silk also contains hormones, steroids, vitamins, and carbs [7,8].
Gram-positive bacteria including Staphylococcus aureus, Bacillus cereus, Bacillus subtilis, and Pseudomonas aeruginosa can't grow in the presence of corn silk, whereas gram-negative bacteria like Shigella sonnei, Shigella flexneri, Enterobacter aerogenes, Salmonella typhi, and Salmonella paratyphi can. Corn silk contains substances that have antimicrobial properties. It specifically has the ability to stop the growth of gram-negative and gram-positive bacteria like Escherichia coli and Staphylococcus aureus [9].
Due to flavonoids' suppression of DNA and RNA production, sweet corn hair has a preference for the gram-positive bacteria it prefers to assault [10]. In order to prevent the growth of S. aureus and E. coli, the tannins included in sweet corn can enter bacterial cells that have been destroyed by saponins and flavonoids. For E. coli and S. aureus, the inhibitory zone diameters are 20 mm and 18 mm, respectively. In another study, sweet corn extract was tested at concentrations of 5, 10, and 15 mg/ml. The results showed that the concentrations of ethanol solvent with sweet corn extract at 10 and 15 mg/ml had a natural effect on preventing bacterial growth [11].
Comparatively to 65 and 75 percent ethanol, 70 percent ethanol solvent is the ideal concentration for dissolving flavonoid compounds [12]. With ethanol, which has a concentration above 70%, total flavonoids decrease [13]. According to research by Nurani et al. (2022) [14], the antibacterial activity of corn hair ethanol extract increased with increasing the concentration of the extract used. The concentrations tested were 10 %, 20%, 30 %, 40 %, 50 %, 60%, 70%, 80%, and 90% with five repetitions each. A larger zone of bacterial inhibition width was visible in maize silk extracts with greater ethanol content. A larger zone of bacterial inhibition width was visible in maize silk extracts with greater ethanol content. With concentrations >70%, the antibacterial activity of corn hair ethanol extract against S. aureus reduced.
This study aims to determine whether sweet corn hair extract can act as a natural disinfectant by lowering bacterial counts and bacterial inhibitory power in milk storage rooms. It also aims to determine the concentration of corn hair extract that has the greatest impact on lowering bacterial counts and bacterial inhibitory power in milk storage rooms.
Experimental sections
Material that will use in the study is as follows: sweet corn, which is harvested at the age of around 70-83 days after planting (HST). Corn silk was obtained in the region of Jatinangor, Sumedang. Ethanol 70%, Alcohol 96%, Nutrients agar (NA). Lactose Broth
Research methods
Study The number of bacteria was suitably determined by RODAC (Replicate Organism Direct Contact), and the disc method was used to measure bacterial inhibition. Three various concentrations of hair extract from corn, including 50 percent, 70 percent, and 90 percent, were examined.Experimental Design and Statistical Analysis
The design test used is Design Random Complete 4 treatments with each 5 times repetition :
P0 = Extract hair corn 0%, (control)
P1 = Extract hair corn 50%
P2 = Extract hair corn 70%
P3 = Extract hair corn 90%
The parameters observed in study this is as following :
1.Initial number bacteria = 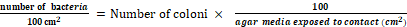
2. The number of bacteria in the milk storage room on the floor and table was measured after spraying
3. Reducing the number of bacteria using the Lukman and Purnawarman formula, ( 2008)
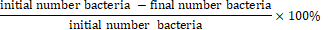
4. Inhibitory Power Bacteria
Research procedure
Preparation of Hair Extraction Corn, Making Corn Hair Extract, Preparing Nutrient Agar Media, Measuring the Number of Bacteria. The RODAC method was used to calculate the quantity of bacteria present on the floor of the milk storage room. This technique determines the number of microorganisms on flat surfaces like tables and floors.
Create reliable NA media.
Place the petri dish on the table and floor for 5 - 10 seconds and cover it with the lid so that those in contact with the surface facing towards on.
Spray the floor with extracted hair corn, which has entered the spray bottle.
Stick the agar on the table and floor that have been treated so that it remains in contact with the floor for 5 - 10 seconds.
Close the cup and incubate for 48 O'clock at 30 - 32℃ on an incubator. The cup
position is not reversed.
Repeat the procedure on every concentration. Extract hair corn gradually on the table and floor before cleaning, the table and floor after cleaning without disinfectant, and the table and floor after cleaning with disinfectant. Growth microbes were observed and calculated.
Colonies growing on NA media were observed using Gram staining after being obtained Gram-positive and harmful bacteria predominantly grow in Petri dishes, followed by measurement of bacterial inhibitory power
13 grams lactose Broth save in tube Erlenmeyer plus 1000 ml of distilled water.
The solution is heated over low heat using a hot plate stirrer and stirred until homogeneous. Close the Erlenmeyer tube and sterilize it using an autoclave for 15 minutes at a temperature 121℃.
take gram-positive and negative bacteria that have grown on media NA with ose, then put it in the solution lactose Broth.
Incubate the bacteria for 24 hours in an incubator and observe changes in lactose Broth become cloudy.
Enter 1 ml of gram-positive and negative bacteria into a test tube containing 15 ml of NA at a temperature of 50℃.
Pour the nutrient agar containing the bacteria into a sterile petri dish and leave it until it solidifies.
Measurement of Bacterial Inhibitory Power
Power activity resistor bacteria extract hair corn be measured using Kirby-Bauer or method diffusion paper disc.
Keep paper disc in solution and extract hair corn on each concentration. For 5 minutes, then drain.
Put paper disc (diameter 6 mm) on Petri dishes containing NA solution and gram-positive bacteria, carried out the same way for gram-negative bacteria.
Incubate the plate for 24 O'clock at a temperature of 37℃.
Count Power inhibitory use period shove.


