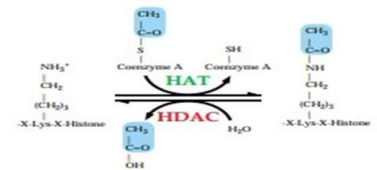
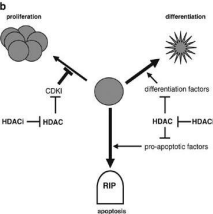
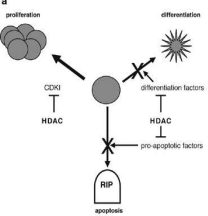
When deacetylation occurs in the normally active region and hyperacetylation occurs in the normal silence region, several diseases can develop. The activity of HAT/HDAC should be regulated, otherwise, dysregulation occurs that leads to various kinds of tumours, cancers, metabolic disorders, neurological disorders, and also the cardiological problem [12].
(i) Cancer:
A set of key genes which influence the growth of the cancer cells and play an important role in their survival are strongly maintained by the HDACs. After knocking out the different HDACs (each was a member of class I HDAC) from individual mice, the importance of HDACs in gene regulation was observed. The observation shows that the mice in which the HDAC1 was absent, died before birth due to proliferation defects. The mice in which HDAC2 was absent, died on the first day after birth due to cardiac malformation. The mice in which the HDAC3 was absent, died before birth due to gastrulation. According to the types of tumours, the levels of HDAC are varied in different types of cancer cells such as HDAC1 are present in prostate, lung, esophageal, colon and breast cancer cells; HDAC2 are present in colorectal, cervical and gastric cancer cells; HDAC3 is expressed in colon and breast tumour cells; HDAC6 are present in mammary tumour cells; HDAC8 is present in neuroblastoma cells and HDAC11 is expressed in rhabdomyosarcoma. Histone H4 hypoacetylation at lysine 16 linked with multiprotein complexes was highlighted as an epigenetic signature in cancer cells. The functions of some proteins which are most important in cancer (tumour protein p53, α3 subunit RUNX3, STAT3, β-catenin, estrogen receptor, EKLF, Myc, MyoD, NF-kB, HIF-1α, GATA family), are modulated by HDACs [13] [27] [28]. Acute myeloid leukaemia and urothelial are observed for low expression of HDAC5 but it can cause breast cancer with high expression. Renal cancer, non-small cell lung cancer, thyroid cancer is caused by the upregulation of HDAC9. Lung cancer and gastric cancer are caused by the low regulation of HDAC10 [25]. Due to the acetylation/deacetylation mechanism of many DNA repair proteins, the pathogenesis of tumours was elucidated which helps to discover the new drugs for post-translational modification for the treatment of cancer [26]. It is published in a recent study that tumour suppression can be influenced by PIB5PA and its downregulation was also observed in melanoma. HDACs can cause histone hypoacetylation which leads to the downregulation of PIB5PA and decreases the survival of melanoma cells [10]. The inflammatory response is increase
in an in vivo allergic inflammatory response model by HDAC1 and the parenchymal lung inflammation can also increase for HDAC1 deficiency in the Th2-type asthma model of mice [8].
(ii) Neurological disease:
Epigenetic changes that occur due to deacetylation by HDAC class I proteins are associated with neurological disease. It was observed from an experiment that the variation of Histone acetylation levels as a result of psychostimulant interaction are affected by HDACs [31]. Critical defects in brain development have been shown due to the deficiency of HDAC4. HDAC9 appears to be important for mature neuron function and deregulation of this can cause defects in brain cells [25]. Huntington's and Alzheimer's are two neurological diseases caused by the imbalance between histone acetylation and deacetylation [32] [33].
(iii) Heart disease:
The deletion of HDAC3 in the heart causes interstitial fibrosis. In the T-cells in the interchange between intestinal lymphocytes and intestinal epithelial cells, the role of HDAC3 has also been observed. A loss of cardiac function has been shown after stress in HDAC5-null mice due to an increase in cardiac hypertrophy [25]. In heart failure, HDACs have been discovered to interact with the transcription factor Mef2 and control gene expression [7].
(iv) Vascular disease:
The epigenetic modification of chromatin has a role in vascular diseases such as Vascular restenosis, atherosclerosis. Studies have indicated that HDAC activity can impact angiogenesis as a result of hypoxia, shear stress and VEGF-induced stem cell differentiation. It is evident that the HDAC7-null cell is involved in the development of normal vascular and in embryonic lethality [6].