Kinetics is a branch of science that handles the mechanisms and rates of any processes – chemical, physical and biological processes. Kinetic studies in microbiology cover all dynamic manifestations of microbial life: growth itself, survival and death, product formation, adaptations, mutations, cell cycles, environmental effects, and biological interactions. Cell growth is the increase in the mass and physical size of a cell which is affected by the physical, biological and chemical factors [1].
Microbial growth kinetics shows the relationship between the specific growth rate (μ) of a microbial population and the substrate concentration (s). It is an indispensable tool in all fields of microbiology, be it physiology, genetics, ecology, or biotechnology, and therefore it is an important part of the basic teaching of microbiology [1]. Growth kinetics is an autocatalytic reaction which implies that the rate of growth is directly proportional to the concentration of cell. Cell division contributes to cell growth, for different microorganism’s cell division is achieved in various ways; Bacteria by binary fission, viruses by intracellular division and yeast by budding [2].
For bacteria, time required to complete a fission cycle is known as doubling/generation time, and as long as condition of environment remains favorable, doubling time remains at a constant rate [3]. Microorganisms used in industrial processes are cultivated in batch, fed-batch and continuous cultures [4,5].
Batch Culture
This is an environment where fixed amount of culture media and growth requirement for desired microorganism is set in a closed system, and when the nutrient is used up, the cell product is extracted from the culture before another batch is put in place. In this system, growth and yield rate is affected by concentration and availability of nutrients. At low nutrient concentration, the rate of growth is reduced and at high nutrient concentration the cell yield continues to increase [6].
Fed-Batch Culture
In this process, nutrients are supplied to the system while the cultivation is ongoing. In this system, there is a base supplement to start up cell cultivation, but subsequently nutrients are added to prevent depletion and decline in cell growth and product formation [6].
Continous Culture
This is an open system whereby there is a simultaneous addition of nutrient to a culture media and removal of products, to achieve a steady rate of growth. This prevents the microorganisms from getting into a stationary phase. Continuous culture methods were developed to grow cells in a constant environment and have been used for decades to study basic microbial physiology in a controlled and reproducible manner [7]. Contrast to a batch culture, in a chemostat, growth rate and yield can be controlled independently [6].
When microorganisms are cultivated, certain growth patterns are observed. Using batch culture system as an example, the following phases are observed during bacterial growth: Lag Phase, Log Phase, Stationary Phase and Death Phase [8].
Bacterial Growth Phase
Lag Phase: This is the initial stage of cultivation in which the cells are adjusting to the environment. No immediate increase is recorded at this phase as most cells do not reproduce immediately but instead synthesize enzymes to utilize nutrients in the medium [9].
Log Phase: Bacteria synthesize the necessary chemicals for conducting metabolism in their new environment, and they then enter a phase of rapid chromosome replication, growth, and reproduction. Population increases logarithmically and reproductive rate reaches a constant as DNA protein syntheses are maximized. Bacteria in the phase are more susceptible to antimicrobial drugs that interfere with metabolism and they are preferred for gram staining because most cell walls are intact. The metabolic rate of individual cells is at a maximum during log phase which is sometimes preferred for industrial and laboratory purposes [8].
Stationary Phase: In this phase, nutrients are depleted and wastes accumulate and the rate of reproduction decreases. The number of dying cells equals the number of cells being produced, and the size of the population becomes stationary. During this phase the metabolic rate of surviving cells declines [10].
Death Phase: If nutrients are not added and wastes are not removed, a population reaches a point at which cells die at a faster rate than they are produced. Such a culture has entered the death phase (or decline phase). During the death phase, some cells remain alive and continue metabolizing and reproducing, but the number of dying cells exceeds the number of new cells produced, so that eventually the population decreases to a fraction of its previous abundance. In some cases, all the cells die, while in others a few survivors may remain indefinitely. The latter case is especially true for cultures of bacteria that can develop resting structures called endospore. Some cells are viable but not culturable (VBNC) that is they are alive, but dormant [8,10].
Batch growth kinetics of a microbe follows a growth curve with lag phase as the initial phase during which cells adapt to a new environment. Multiple lag phases occur if the media is supplemented with more than one sugar and such type of growth is referred to as diauxic growth. Following the lag phase is the log phase in which the cell mass and cell number increases exponentially and then the depletion of nutrients starts which indicates the deceleration phase. The accumulation of toxic products results in deceleration phase after which stationary phase commences in which growth rate equals the death rate. The continuous growth kinetics accessed by a perpetual feeding process in which the growth is controlled by the concentration of the rate limiting nutrient [9].
The growth kinetics explains the relationship between the specific growth rate of a microbe and its substrate concentration. Microbial growth kinetics largely depends on the laboratory culture conditions. In batch culture, microbial cell composition and its state change as a function of time and thus the rate of increase in biomass concentration was monitored [10].
Alternatively, in continuous culture the concentration of substrate is at equilibrium and the culture grows at stable physiological state which provides more precise and reproducible data. However, the constant growth conditions represent an artificial growth environment which does not explain many microbial kinetic phenomena. Thus, growth of microbial cells was performed under mixed substrates rather than single substrate to understand the growth kinetics of microorganisms in their natural environment [11]. The substrate such as nutrients (carbon and nitrogen sources), hormones and growth factors influence the growth pattern of microbial and mammalian cells. Substrate limited and substrate-sufficient growth would be observed on the basis of the relative availability of the substrate
Measurement of Microbial Growth
Direct Methods such as viable plate counts, membrane filtration, microscopic counts, and the most probable number method [12].
Viable Plate Count:
This is done by using the spread and pour plate techniques, whereby the diluted sample of bacteria is spread over solid agar surface or mixed with agar and poured into Petri plate, after incubation the numbers of organisms are determined by counting the number of colonies multiplied by the dilution factors. The results are expressed as colony forming units (CFU) [13].
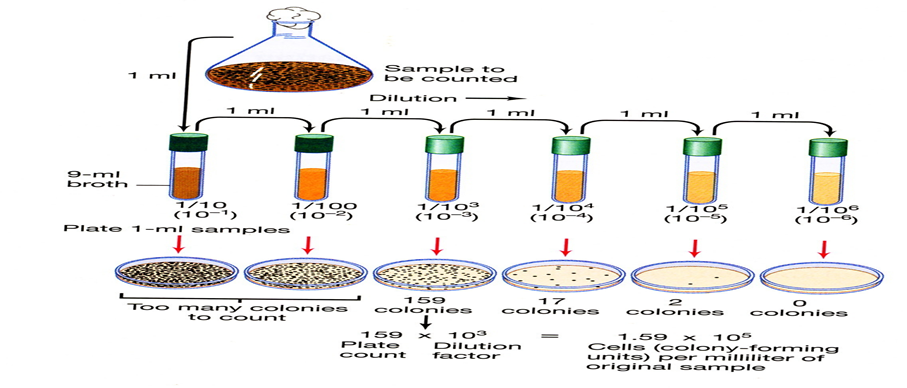
Viable Plate Count (Black, 2015).[14]
Membrane Filtration:
In this method, a large samples are poured through a membrane filter with pores small enough to trap the cells, after which the membrane is transferred onto a solid medium, and the colonies present after incubation are counted. In this case, the number of colonies is equal to the number of CFUs in the original large sample [15].
Most Probable Number:
This as a statistical estimation technique based on the more bacteria in a sample, the more dilutions are required to reduce their number to zero (Black, 2015). [14]
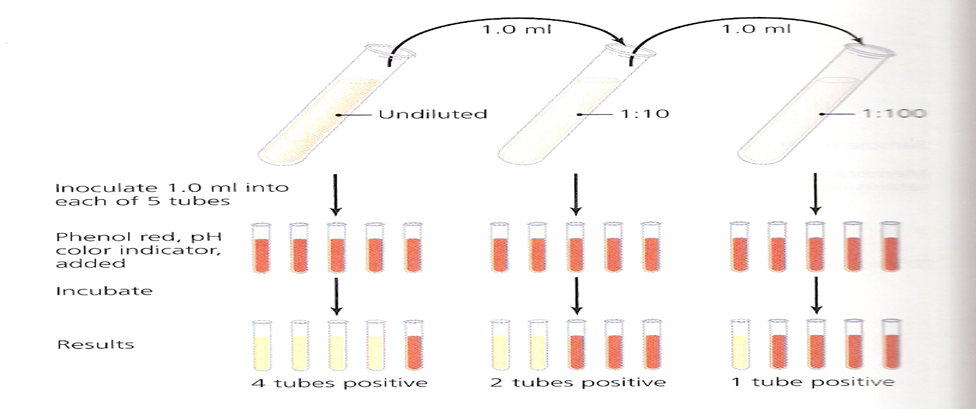
Most Probable Number (Hogg, 2013) [8]
Indirect Methods such as dry weight and turbidity
Dry Weight:
Some microorganisms, particularly filamentous microorganisms which are difficult to measure by direct methods are filtered from their culture medium, dried, and weighed. The dry weight method is suitable for broth cultures, but growth cannot be followed over time because the organisms are killed during the process (Black, 2015). [14]
Turbidity:
As bacteria reproduce in a broth culture, the broth often becomes turbid (cloudy). An indirect method for estimating the growth of a microbial population involves measuring changes in turbidity using a device called a spectrophotometer [13]. However, there are debates on whether color of the cell growth will affect the results to give an accurate measurement.
Growth Kinetics
Growth kinetics is an autocatalytic reaction which implies that the rate of growth is directly proportional to the concentration of cell. The cell concentration is measured by direct and indirect methods. Microbial growth kinetics explains the relationship between the specific growth rate of a microbe and its substrate concentration. Microbial growth kinetics largely depends on the laboratory culture conditions [16].In batch culture, microbial cell composition and its state change as a function of time and thus the rate of increase in biomass concentration is monitored.
Alternatively, in continuous culture the concentration of substrate is at equilibrium and the culture grows at stable physiological state which provides more precise and reproducible data.The kinetic model of cell growth is substantially capable to predict product formation. Mathematical models provide a strategy for solving problems encountered in fermentation process [9].
Growth kinetics is classified into three based on the relationship between product synthesis and energy generation in the cell:
Growth associated: Growth linked products are formed by growing cells and hence primary metabolites. Product is formed simultaneously with growth of cells. That is product concentration increases with cell concentration.
Non-growth associated: They are formed by cells which are not metabolically active and hence are called secondary metabolites. Product formation is unrelated to growth rate but is a function of cell concentration.
Mixed-growth associated: The product formation from the microorganism depends on both growth and non-growth associated. It takes place during growth and stationary phases. Product formation is a combination of growth rate and cell concentration [2].
A mathematical relationship exists between the number of cells present in a culture initially and the number present after a period of exponential growth:
N = N02n
Where: N is the final cell number; No is the initial cell number and n is the number of generations that have occurred during the period of exponential growth [15].
The generation time (g) of the exponentially growing population is (t/n). Where: t is the duration of exponential growth expressed in days, hours, or minutes, depending on the organism and the growth conditions.
From knowledge of the initial and final cell numbers in an exponentially growing cell population, it is possible to calculate n, and from n and knowledge of t, the generation time g can be calculated [15].
Concept of Microbial Death
The rate of microbial death is used to develop standard protocols for sterilization in many industries. The goal is to find out what is the minimum time needed to achieve acceptable level of sterilization for a specific purpose. The killing agent can be different (e.g., heat, chemical with certain concentration) depending on the specific application. When the killing factor is heat, the phrase thermal death can be used. Thermal death time is a concept used to determine how long it takes to kill a specific bacterium at a specific temperature. It was originally developed for food canning and has found applications in cosmetics, and in producing salmonella-free feeds for animals (e.g. poultry, and pharmaceuticals). In the food industry, thermal processing helps reduce the number of bacteria in the product. Time-temperature measurements of bacterial reduction are determined by a D-value, meaning how long it would take to reduce the bacterial population by 90%. Z-value is used to determine the time values with different D-values at different temperatures. This D-value is affected by pH of the product where low pH has faster D values on various foods [15].




