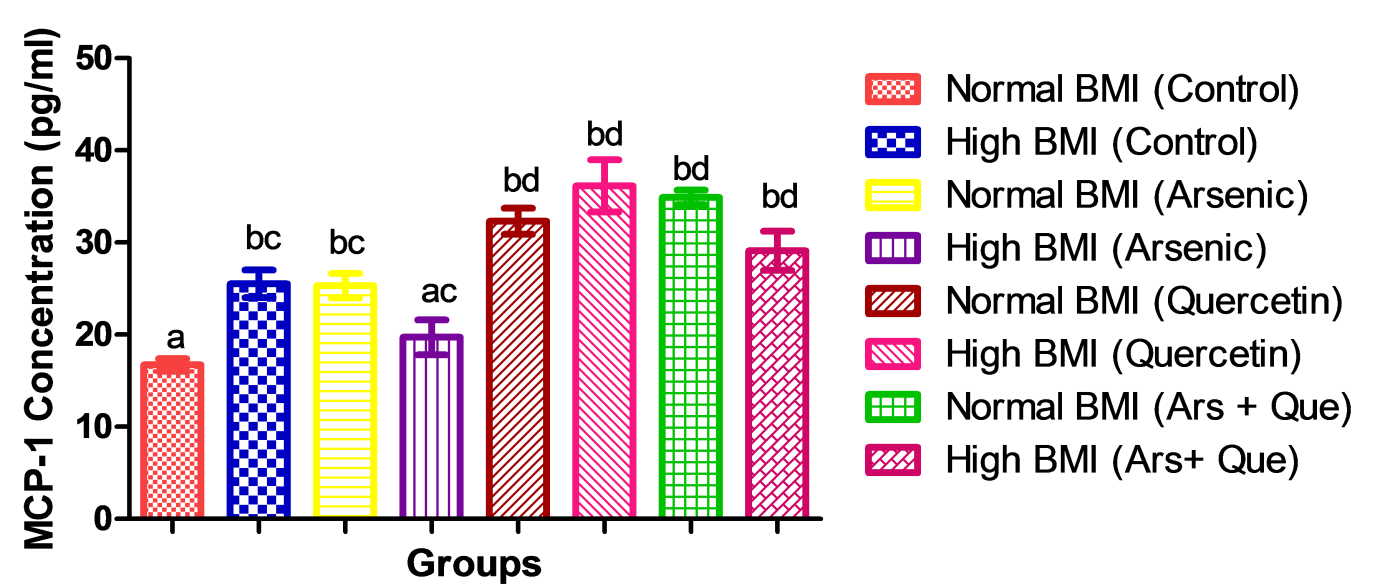
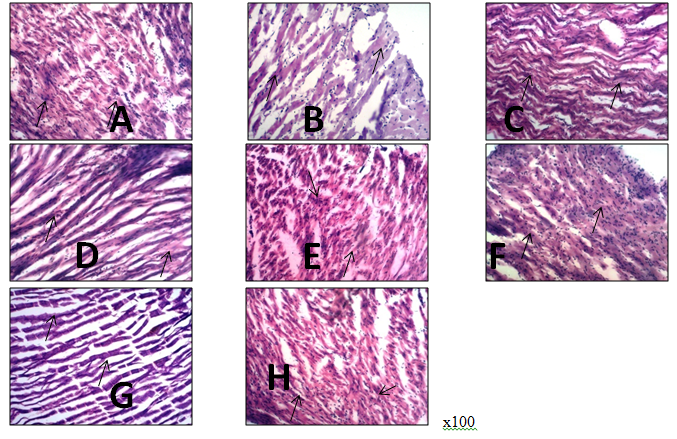
Abdelkarem, H.M. and L.H. Fadda. "Flaxseed and Quercetin Improve Anti-Inflammatory Cytokine Level and Insulin Sensitivity in an Animal Model of Metabolic Syndrome, the Fructose-Fed Rats." Arabian Journal of Chemistry, 2013, DOI: 10.1016/j.arabjc.2013.11.042.
Afolabi, O.K. et al. "Assessment of Lipid Peroxidation Markers and Proinflammatory Cytokines in Arsenite-Exposed Rats." Asian Journal of Natural and Applied Sciences, vol. 2, no. 2, 2013, pp. 8–18.
Al-Aubaidy, H.A. and H.F. Jelinek. "8-Hydroxy-2-Deoxy-Guanosine Identifies Oxidative DNA Damage in a Rural Prediabetes Cohort." Redox Report, vol. 15, 2010, pp. 155–160.
Albuali, W.H. "Evaluation of Oxidant-Antioxidant Status in Overweight and Morbidly Obese Saudi Children." World Journal of Clinical Pediatrics, vol. 3, no. 1, 2014, pp. 6–13.
Al-Forkan, M. et al. "A Sub-Chronic Exposure Study of Arsenic on Haematological Parameters, Liver Enzyme Activities, Histological Studies and Accumulation Pattern of Arsenic in Organs of Wistar Albino Rats." Journal of Cytology and Histology, 2016, doi:10.4172/2157-7099.S5-006.
Aliyu, M. et al. "Ethanol Suppresses the Effects of Sodium Arsenate in Male Wistar Albino Rats." Scientific Reports, vol. 1, 2012, pp. 1–6.
Askari, G. et al. "The Effect of Quercetin Supplementation on Selected Markers of Inflammation and Oxidative Stress." Journal of Research in Medical Sciences, vol. 17, no. 7, 2012, pp. 637–641.
Avwioro, O.G. "Histochemistry and Tissue Pathology: Principle and Techniques." Claverianum Journal, vol. 15, no. 8, 2001, pp. 45–77.
Beatty, E.R. et al. "Effect of Dietary Quercetin on Oxidative DNA Damage in Healthy Human Subjects." British Journal of Nutrition, vol. 84, 2000, pp. 919–925.
Black, P.H. "The Inflammatory Response Is an Integral Part of the Stress Response: Implications for Atherosclerosis, Insulin Resistance, Type II Diabetes and Metabolic Syndrome X." Brain Behavior and Immunity, vol. 17, 2003, pp. 350–364.
Boots, A.W. et al. "In Vitro and Ex Vivo Anti-Inflammatory Activity of Quercetin in Healthy Volunteers." Nutrition, vol. 24, 2008, pp. 703–710.
Boyle, S.P. et al. "Bioavailability and Efficiency of Rutin as an Antioxidant: A Human Supplementation Study." European Journal of Clinical Nutrition, vol. 54, 2000, pp. 774–782.
Bruguera, M. et al. "Incidence and Clinical Significance of Sinusoidal Dilatation in Liver Biopsies." Gastroenterology, vol. 75, 1978, pp. 474–478.
Butterfield, D.A. et al. "Evidence That Amyloid Beta-Peptide–Induced Lipid Peroxidation and Its Sequelae in Alzheimer’s Disease Brain Contribute to Neuronal Death." Neurobiology of Aging, vol. 23, 2002, pp. 655–664.
Chang, S.I. et al. "Arsenic-Induced Toxicity and the Protective Role of Ascorbic Acid in Mouse Testis." Toxicology and Applied Pharmacology, vol. 218, 2007, pp. 196–203.
Chen, Y. et al. "Glutathione Defense Mechanism in Liver Injury: Insights from Animal Models." Food and Chemical Toxicology, vol. 60, 2013, pp. 38–44.
Chen, H. et al. "Chronic Inorganic Arsenic Exposure Induces Hepatic Global and Individual Gene Hypomethylation: Implications for Arsenic Hepatocarcinogenesis." Carcinogenesis, vol. 25, 2004, pp. 1779–1786.
Chen, L. et al. "Binding Interaction of Quercetin-3-Beta-Galactoside and Its Synthetic Derivatives with SARS-CoV 3CL(pro): Structure-Activity Relationship Studies Reveal Salient Pharmacophore Features." Bioorganic and Medicinal Chemistry, vol. 14, 2006, pp. 8295–8306.
Chen, Y. et al. "Arsenic Exposure from Drinking Water and Mortality from Cardiovascular Disease in Bangladesh: Prospective Cohort Study." BMJ Journals, vol. 342, 2011, Article ID d2431.
Cheng, T.J. et al. "The Association Between Arsenic Exposure from Drinking Water and Cerebrovascular Disease Mortality in Taiwan." Water Research, vol. 44, 2010, pp. 5770–5776.
Chiang, L.C. et al. "In Vitro Antiviral Activities of Caesalpinia Pulcherrima and Its Related Flavonoids." Journal of Antimicrobial Chemotherapy, vol. 52, 2003, pp. 194–198.
Choi, S. et al. "Anti-Inflammatory Effect of 8-Hydroxy-20-Deoxyguanosine on Lipopolysaccharide-Induced Inflammation via Rac Suppression in BALB/c Mice." Free Radical Biology & Medicine, vol. 43, 2007, pp. 1594–1603.
Ciz, M. et al. "The Influence of Wine Polyphenols on Reactive Oxygen and Nitrogen Species Production by Murine Macrophages RAW 264.7." Physiological Research, vol. 57, 2008, pp. 393–402.
Comalada, M. et al. "Inhibition of Pro-Inflammatory Markers in Primary Bone Marrow-Derived Mouse Macrophages by Naturally Occurring Flavonoids: Analysis of the Structure-Activity Relationship." Biochemical Pharmacology, vol. 72, 2006, pp. 1010–1021.
Comalada, M. et al. "In Vivo Quercitrin Anti-Inflammatory Effect Involves Release of Quercetin, Which Inhibits Inflammation Through Down-Regulation of the NF-KappaB Pathway." European Journal of Immunology, vol. 35, 2005, pp. 584–592.
Coskun, O. et al. "Quercetin, a Flavonoid Antioxidant, Prevents and Protects Streptozotocin-Induced Oxidative Stress and Beta-Cell Damage in Rat Pancreas." Pharmacological Research, vol. 51, 2005, pp. 117–123.
Coussens, L.M. and Z. Werb. "Inflammation and Cancer." Nature, vol. 420, 2002, pp. 860–867.
Coyle, J.T. and P. Puttfarken. "Oxidative Stress, Glutamate and Neurodegenerative Disorders." Science, vol. 262, 1993, pp. 689–695.
Cushnie, T.P. and A.J. Lamb. "Antimicrobial Activity of Flavonoids." International Journal of Antimicrobial Agents, vol. 26, 2005, pp. 343–356.
Dangleben, N.L. et al. "Arsenic Immunotoxicity: A Review." Environmental Health, vol. 12, no. 1, 2013, p. 73.
Das, N. et al. "Arsenic Exposure Through Drinking Water Increases the Risk of Liver and Cardiovascular Diseases in the Population of West Bengal, India." BMC Public Health, vol. 12, 2012, Article ID 639.
Das, S. et al. "Sodium Arsenite Mediated Immunodisruption Through Alteration of Transcription Profile of Cytokines in Chicken Splenocytes Under In Vitro System." Molecular Biology Reports, vol. 38, 2011, pp. 171–176.
Davis, J.M. et al. "Quercetin Increases Brain and Muscle Mitochondrial Biogenesis and Exercise Tolerance." American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, vol. 296, 2009, pp. R1071–R1077.
Davis, J.M. et al. "Quercetin Reduces Susceptibility to Influenza Infection Following Stressful Exercise." American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, vol. 295, 2008, pp. R505–R509.
de Boer, V.C. et al. "Chronic Quercetin Exposure Affects Fatty Acid Catabolism in Rat Lung." Cellular and Molecular Life Sciences, vol. 63, 2006, pp. 2847–2858.
Dias, A.S. et al. "Quercetin Decreases Oxidative Stress, NF-KappaB Activation and iNOS Overexpression in Liver of Streptozotocin-Induced Diabetic Rats." Journal of Nutrition, vol. 35, 2005, pp. 2299–2304.
Dimova, S. et al. "Safety-Assessment of 3-Methoxyquercetin as an Antirhinoviral Compound for Nasal Application: Effect on Ciliary Beat Frequency." International Journal of Pharmaceutics, vol. 263, 2003, pp. 95–103.
Dludla, P. et al. "Inflammation and Oxidative Stress in an Obese State and the Protective Effects of Gallic Acid." Nutrients, vol. 11, 2019, Article ID 23.
Duramad, P. et al. "Cytokines and Other Immunological Biomarkers in Children’s Environmental Health Studies." Toxicology Letters, vol. 172, 2007, pp. 48–59.
Edwards, R.L. et al. "Quercetin Reduces Blood Pressure in Hypertensive Subjects." Journal of Nutrition, vol. 137, 2007, pp. 2405–2411.
Egert, S. et al. "Quercetin Reduces Systolic Blood Pressure and Plasma Oxidized Low-Density Lipoprotein Concentrations in Overweight Subjects with a High-Cardiovascular Disease Risk Phenotype: A Double-Blinded, Placebo-Controlled Cross-Over Study." British Journal of Nutrition, vol. 102, 2009, pp. 1065–1074.
Egert, S. et al. "Daily Quercetin Supplementation Dose-Dependently Increases Plasma Quercetin Concentrations in Healthy Humans." Journal of Nutrition, vol. 138, 2008, pp. 1615–1621.
Eick, S.M. and C. Steimaus. "Arsenic and Obesity: A Review of Causation and Interaction." Current Environmental Health Reports, vol. 7, no. 21, 2020, pp. 1–9.
Engel, R.R. and A.H. Smith. "Arsenic in Drinking Water and Mortality from Vascular Disease: An Ecologic Analysis in 30 Counties in the United States." Archives of Environmental Health, vol. 49, 1994, pp. 418–427.
Erdman, J.W. et al. "Flavonoids and Heart Health: Proceedings of the ILSI North America Flavonoids Workshop." Journal of Nutrition, vol. 137, 2005, pp. 718S–737S.
Flora, S.J. "Arsenic-Induced Oxidative Stress and Its Reversibility." Free Radical Biology and Medicine, vol. 51, 2011, pp. 257–281.
Fry, R.C. et al. "Activation of Inflammation/NF-KappaB Signaling in Infants Born to Arsenic-Exposed Mothers." PLoS Genetics, vol. 3, 2007, Article ID e207.
Galkina, E. and K. Ley. "Vascular Adhesion Molecules in Atherosclerosis." Arteriosclerosis, Thrombosis and Vascular Biology, vol. 27, 2007, pp. 2292–2301.
Ghosh, A. et al. "Hepatoprotective and Neuroprotective Activity of Liposomal Quercetin in Combating Chronic Arsenic-Induced Oxidative Damage in Liver and Brain of Rats." Drug Delivery, vol. 18, 2011, pp. 451–459.
Gomez-Rubio, P. et al. "Association Between Body Mass Index and Arsenic Methylation Efficiency in Adult Women from Southwest U.S. and Northwest Mexico." Toxicology and Applied Pharmacology, vol. 252, 2011, pp. 176–182.
Gong, M. et al. "Quercetin Up-Regulates Paraoxonase 1 Gene Expression with Concomitant Protection Against LDL Oxidation." Biochemical and Biophysical Research Communications, vol. 379, 2009, pp. 1001–1004.
Hall, M. et al. "Determinants of Arsenic Metabolism: Blood Arsenic Metabolites, Plasma Folate, Cobalamin and Homocysteine Concentrations in Maternal-Newborn Pairs." Environmental Health Perspectives, vol. 115, 2007, pp. 1503–1509.
Halliwell, B. "Oxidative Stress and Neurodegeneration: Where Are We Now?" Journal of Neurochemistry, vol. 97, no. 6, 2006, pp. 1634–1658.
Harwood, M. et al. "A Critical Review of the Data Related to the Safety of Quercetin and Lack of Evidence of In Vivo Toxicity, Including Lack of Genotoxic/Carcinogenic Properties." Food and Chemical Toxicology, vol. 45, 2007, pp. 2179–2205.
Heinecke, J.W. "Oxidants and Antioxidants in the Pathogenesis of Atherosclerosis: Implications for the Oxidized Low-Density Lipoprotein Hypothesis." Atherosclerosis, vol. 141, 1998, pp. 1–15.
Higa, J.K. et al. "Supplement of Bamboo Extract Lowers Serum Monocyte Chemoattractant Protein-1 Concentration in Mice Fed a High-Fat Diet." British Journal of Nutrition, vol. 106, 2011, pp. 1810–1813.
Hoek-van den Hil, E.F. et al. "Quercetin Induces Hepatic Lipid Omega-Oxidation and Lowers Serum Lipid Levels in Mice." PLoS One, vol. 8, 2013, DOI: 10.1371/journal.pone.0051588.
Hou, L., Zhou, B., Yang, L. and Z.L. Liu. "Inhibition of Human Low-Density Lipoprotein Oxidation by Flavonols and Their Glycosides." Chemistry and Physics of Lipids, vol. 129, 2004, pp. 209–219.
Hughes, M.F. et al. "Arsenic Exposure and Toxicology: A Historical Perspective." Toxicological Sciences, vol. 123, no. 2, 2011, pp. 305–332.
Iiyama, K. et al. "Patterns of Vascular Cell Adhesion Molecule-1 and Intercellular Adhesion Molecule-1 Expression in Rabbit and Mouse Atherosclerotic Lesions and at Sites Predisposed to Lesion Formation." Circulation Research, vol. 85, 1999, pp. 199–207.
Irie, M. et al. "Occupational and Lifestyle Factors & Urinary 8-Hydroxydeoxyguanosine." Cancer Science, vol. 96, 2005, pp. 600–606.
Jomova, K. et al. "Arsenic: Toxicity, Oxidative Stress and Human Disease." Journal of Applied Toxicology, vol. 31, no. 2, 2010, pp. 95–107.
Jung, C.H. et al. "Quercetin Reduces High-Fat Diet-Induced Fat Accumulation in the Liver by Regulating Lipid Metabolism Genes." Phytotherapy Research, vol. 27, 2013, pp. 139–143.
Justino, G.C. et al. "Plasma Quercetin Metabolites: Structure-Antioxidant Activity Relationships." Archives of Biochemistry and Biophysics, vol. 432, 2004, pp. 109–121.
Karim, M.R. et al. "Increases in Oxidized Low-Density Lipoprotein and Other Inflammatory and Adhesion Molecules with a Concomitant Decrease in High-Density Lipoprotein in Individuals Exposed to Arsenic in Bangladesh." Toxicological Sciences, vol. 135, 2013, pp. 17–25.
Kasai, H. "Analysis of a Form of Oxidative DNA Damage, 8-Hydroxy-2-Deoxyguanosine, as a Marker of Cellular Oxidative Stress During Carcinogenesis." Mutation Research, vol. 387, 1997, pp. 147–163.
Kelly, A.S. et al. "Relation of Circulating Oxidized LDL to Obesity and Insulin Resistance in Children." Pediatric Diabetes, vol. 11, 2010, pp. 552–555.
Kobori, M. et al. "Chronic Dietary Intake of Quercetin Alleviates Hepatic Fat Accumulation Associated with Consumption of a Western-Style Diet in C57/BL6 J Mice." Molecular Nutrition and Food Research, vol. 55, 2011, pp. 530–540.
Koehler, Y. et al. "Uptake and Toxicity of Arsenite and Arsenate in Cultured Brain Astrocytes." Journal of Trace Elements in Medicine and Biology, vol. 28, 2014, pp. 328–337.
Krzysztoszek, J. et al. "Obesity: An Analysis of Epidemiological and Prognostic Research." Archives of Medical Sciences, vol. 11, 2015, pp. 24–33.
Lee, H. et al. "Obesity, Inflammation and Diet." Pediatric Gastroenterology, Hepatology and Nutrition, vol. 16, 2013, pp. 143–152.
Lee, K.M. et al. "Protective Effect of Quercetin Against Arsenite-Induced COX-2 Expression by Targeting PI3K in Rat Liver Epithelial Cells." Journal of Agricultural and Food Chemistry, vol. 58, 2010, pp. 5815–5820.
Leemans, Jan C., et al. "Pattern Recognition Receptors and the Inflammasome in Kidney Disease." Nature Reviews Nephrology, vol. 10, 2014, pp. 398–414.
Lessner, Susan M., et al. "Atherosclerotic Lesions Grow through Recruitment and Proliferation of Circulating Monocytes in a Murine Model." American Journal of Pathology, vol. 160, 2002, pp. 2145–2155.
Li, H. et al. "Arteriosclerosis Thrombosis." Arteriosclerosis Thrombosis, vol. 13, 1993, pp. 197–203.
Libby, Peter. "Inflammation in Atherosclerosis." Nature, vol. 420, 2002, pp. 868–874.
Libby, Peter, et al. "Circulation." Circulation, vol. 105, 2002, pp. 1135–1142.
Lindberg, Anna-Lena, et al. "Metabolism of Low-Dose Inorganic Arsenic in a Central European Population: Influence of Sex and Genetic Polymorphisms." Environmental Health Perspectives, vol. 115, 2007, pp. 1081–1086.
Liu, Y. J. et al. "Alterations of nAChRs and ERK1/2 in the Brains of Rats with Chronic Fluorosis and Their Connections with the Decreased Capacity of Learning and Memory." Toxicology Letters, vol. 192, 2010, pp. 324–329.
Loke, W. M., et al. "Quercetin and Its In Vivo Metabolites Inhibit Neutrophil-Mediated Low-Density Lipoprotein Oxidation." Journal of Agricultural and Food Chemistry, vol. 56, 2008, pp. 3609–3615.
Mandal, B. K., and K. T. Suzuki. "Arsenic Round the World: A Review." Talanta, vol. 58, 2002, pp. 201–235.
Manna, S. K., et al. "Resveratrol Suppresses TNF-Induced Activation of Nuclear Transcription Factors NF-kappa B, Activator Protein-1 and Apoptosis: Potential Role of Reactive Oxygen Intermediates and Lipid Peroxidation." Journal of Immunology, vol. 164, 2000, pp. 6509–6519.
Marseglia, L., et al. "Oxidative Stress in Obesity: A Critical Component in Human Diseases." International Journal of Molecular Sciences, vol. 16, 2015, pp. 378–400.
Martinez, V. D. et al. "Arsenic Exposure and the Induction of Human Cancers." Journal of Toxicology, 2011, Article ID 431287.
Martín-Gallán, P., et al. "Changes in Oxidant-Antioxidant Status in Young Diabetic Patients from Clinical Onset Onwards." Journal of Cellular and Molecular Medicine, vol. 11, 2007, pp. 1352–1366.
Marui, N., et al. "Vascular Cell Adhesion Molecule-1 (VCAM-1) Gene Transcription and Expression Are Regulated through an Antioxidant-Sensitive Mechanism in Human Vascular Endothelial Cells." Journal of Clinical Investigation, vol. 92, 1993, pp. 1866–1874.
Medrano, M. A., et al. "Arsenic in Public Water Supplies and Cardiovascular Mortality in Spain." Environmental Research, vol. 110, 2010, pp. 448–454.
Miller, W. H., Jr., et al. "Mechanisms of Action of Arsenic Trioxide." Cancer Research, vol. 62, 2002, pp. 3893–3903.
Moon, J. H., et al. "Identification of Quercetin 3-O-beta-D-glucuronide as an Antioxidative Metabolite in Rat Plasma after Oral Administration of Quercetin." Free Radical Biology & Medicine, vol. 30, 2001, pp. 1274–1285.
Moon, K.A. et al. "Association Between Exposure to Low to Moderate Arsenic Levels and Incident Cardiovascular Disease: A Prospective Cohort Study." Annals of Internal Medicine, vol. 159, 2013, pp. 649–659.
Morgan, M.J. and Z.G. Liu. "Crosstalk of Reactive Oxygen Species and NF-kappaB Signaling." Cell Research, vol. 21, 2011, pp. 103–115.
Nair, M.P. et al. "The Flavonoid Quercetin Inhibits Proinflammatory Cytokine (Tumor Necrosis Factor Alpha) Gene Expression in Normal Peripheral Blood Mononuclear Cells via Modulation of the NF-kappa Beta System." Clinical and Vaccine Immunology, vol. 13, 2006, pp. 319–328.
National Research Council. Critical Aspects of EPA's IRIS Assessment of Inorganic Arsenic: Interim Report. 2014, www.nap.edu/catalog.php?record_id=18594. Accessed 2 June 2014.
Naujokas, M.F. et al. "The Broad Scope of Health Effects from Chronic Arsenic Exposure: Update on a Worldwide Public Health Problem." Environmental Health Perspectives, vol. 121, 2013, pp. 295–302.
Nelken, N.A. et al. "Monocyte Chemoattractant Protein-1 in Human Atheromatous Plaques." Journal of Clinical Investigation, vol. 88, 1991, pp. 1121–1127.
Neuhouser, M.L. "Dietary Flavonoids and Cancer Risk: Evidence from Human Population Studies." Nutrition and Cancer, vol. 50, 2004, pp. 1–7.
Niraj, P. et al. "Male Reproductive Effect of Arsenic in Mice." Biometals, vol. 14, 2001, pp. 113–117.
Noman, A.S.M. et al. "Arsenic-Induced Histological Alterations in Various Organs of Mice." Journal of Cytology and Histology, vol. 6, 2015, p. 323.
Nutrient Data Laboratory (US). USDA Database for the Flavonoid Content of Selected Foods. Beltsville, MD: US Department of Agriculture, Agricultural Research Service, Beltsville Human Nutrition Research Center, Nutrient Data Laboratory, 2007.
Odbayar, T.O. et al. "Comparative Studies of Some Phenolic Compounds (Quercetin, Rutin and Ferulic Acid) Affecting Hepatic Fatty Acid Synthesis in Mice." Journal of Agricultural and Food Chemistry, vol. 54, 2006, pp. 8261–8265.
Othman, Z.A. et al. "Anti-Atherogenic Effects of Orlistat on Obesity-Induced Vascular Oxidative Stress Rat Model." Antioxidants, vol. 10, 2021, p. 251.
Oyewo, E.B. et al. "Amelioration Potentials of Yoyo Bitters and Aqueous Leaf Extract of Moringa Oleifera in Arsenite-Induced Inflammatory Dysfunctions in Male Wistar Rats." Asian Journal of Medicine and Health, vol. 7, no. 4, 2017, pp. 1–15.
Peng, J. et al. "Stress Proteins as Biomarkers of Oxidative Stress: Effects of Antioxidant Supplements." Free Radical Biology & Medicine, vol. 28, 2000, pp. 1598–1606.
Pergola, G. and F.G. Silvestris. "Obesity as a Major Risk Factor for Cancer." Journal of Obesity, vol. 2013, 2013.
Quindry, J.C. et al. "Oral Quercetin Supplementation and Blood Oxidative Capacity in Response to Ultramarathon Competition." International Journal of Sport Nutrition and Exercise Metabolism, vol. 18, 2008, pp. 601–616.
Rahman, M.M. et al. "Chronic Exposure of Arsenic via Drinking Water and Its Adverse Health Impacts on Humans." Environmental Geochemistry and Health, vol. 31, no. S1, 2009, pp. 189–200.
Read, M.A. “Flavonoids: Naturally Occurring Anti-Inflammatory Agents.” American Journal of Pathology, vol. 147, 1995, pp. 235–237.
Reagan-Shaw, S. et al. “Dose Translation from Animal to Human Studies Revisited.” Federation of American Societies for Experimental Biology Journal, vol. 22, no. 3, 2008, pp. 659–661.
Reichard, J.F. et al. "Long-Term Low-Dose Arsenic Exposure Induces Loss of DNA Methylation.” Biochemical and Biophysical Research Communications, vol. 352, 2007, pp. 188–192.
Rice-Evans, C.A. et al. "Structure-Antioxidant Activity Relationships of Flavonoids and Phenolic Acids.” Free Radical Biology and Medicine, vol. 20, 1996, pp. 933–956.
Rollins, B.J. “Chemokines.” Blood, vol. 90, 1997, pp. 909–928.
Sampson, L. et al. “Flavonol and Flavone Intakes in US Health Professionals.” Journal of the American Dietetic Association, vol. 102, 2002, pp. 1414–1420.
Samuel, E.S. and A.O. Adewale. “Alterations in Some Vital Tissues of Male Wistar Rats Exposed Individually or Simultaneously to Lead Acetate and Sodium Arsenite.” Nigerian Research Journal of Chemical Sciences, vol. 7, 2019, pp. 168–182.
Santra, A. et al. “Hepatic Damage Caused by Chronic Arsenic Toxicity in Experimental Animals.” Journal of Toxicology: Clinical Toxicology, vol. 38, 2000, pp. 395–405.
Sartipy, P. and D.J. Loskutoff. “Monocyte Chemoattractant Protein 1 in Obesity and Insulin Resistance.” Proceedings of the National Academy of Sciences of the United States of America, vol. 100, 2003, pp. 7265–7270.
Selgrade, M.K. “Immunotoxicity: The Risk Is Real.” Toxicological Sciences, vol. 100, 2007, pp. 328–332.
Shanley, R.A. et al. “Quercetin Supplementation Does Not Alter Antioxidant Status in Humans.” Free Radical Research, vol. 44, 2010, pp. 224–231.
Singh, M.K. et al. “Immunomodulatory Role of Emblica officinalis in Arsenic-Induced Oxidative Damage and Apoptosis in Thymocytes of Mice.” BMC Complementary and Alternative Medicine, vol. 13, 2013, p. 193.
Singh, N. et al. “Adverse Health Effects Due to Arsenic Exposure: Modification by Dietary Supplementation of Jaggery in Mice.” Toxicology and Applied Pharmacology, vol. 242, no. 3, 2010, pp. 247–255.
Steinberg, D. “Lewis A. Conner Memorial Lecture: Oxidative Modification of LDL and Atherogenesis.” Circulation, vol. 95, 1997, pp. 1062–1071.
Steinmaus, C. et al. “Obesity and Excess Weight in Early Adulthood and High Risks of Arsenic-Related Cancer in Later Life.” Environmental Research, vol. 142, 2015, pp. 594–601.
Stewart, L. et al. “Quercetin Transiently Increases Energy Expenditure but Persistently Decreases Circulating Markers of Inflammation in C57BL/6 J Mice Fed a High-Fat Diet.” Metabolism, vol. 57, no. 7 Suppl. 1, 2008, pp. S39–S46.
Stewart, L. et al. “Failure of Dietary Quercetin to Alter the Temporal Progression of Insulin Resistance Among Tissues of C57BL/6 J Mice During the Development of Diet-Induced Obesity.” Diabetologia, vol. 52, 2009, pp. 514–523.
Sun, X. et al. “Arsenic Affects Inflammatory Cytokine Expression in Gallus gallus Brain Tissues.” BMC Veterinary Research, vol. 13, 2017, p. 157.
Tondel, M. et al. “Urinary 8-Hydroxydeoxyguanosine in Belarussian Children Relates to Urban Living Rather Than Radiation Dose After the Chernobyl Accident: A Pilot Study.” Archives of Environmental Contamination and Toxicology, vol. 48, 2005, pp. 515–519.
Tsuruya, K. et al. “Accumulation of 8-Oxoguanine in the Cellular DNA and the Alteration of the OGG1 Expression During Ischemia–Reperfusion Injury in the Rat Kidney.” DNA Repair, vol. 2, 2003, pp. 211–229.
Vahter, M. “Health Effects of Early Life Exposure to Arsenic.” Basic and Clinical Pharmacology and Toxicology, vol. 102, 2008, pp. 204–211.
Vrijsen, R. et al. “Antiviral Activity of Flavones and Potentiation by Ascorbate.” Journal of General Virology, vol. 69, 1988, pp. 1749–1751.
Wein, S. et al. “Quercetin Enhances Adiponectin Secretion by a PPAR-Gamma Independent Mechanism.” European Journal of Pharmaceutical Sciences, vol. 41, 2010, pp. 16–22.
World Health Organization. Guidelines for Drinking-Water Quality. 3rd ed., vol. 1: Recommendations, WHO Press, Geneva, 2008.
Wu, L.L. et al. “Urinary 8-OHdG: A Marker of Oxidative Stress to DNA and a Risk Factor for Cancer, Atherosclerosis and Diabetes.” Clinica Chimica Acta, vol. 339, 2004, pp. 1–9.
Wu, M.M. et al. “Gene Expression of Inflammatory Molecules in Circulating Lymphocytes from Arsenic-Exposed Human Subjects.” Environmental Health Perspectives, vol. 111, 2003, pp. 1429–1438.
Xu, M. et al. “Oxidative Damage Induced by Arsenic in Mice/Rats: A Systemic Review and Meta-Analysis.” Biological Trace Element Research, vol. 176, 2017, pp. 154–175.
Yaxiong, X. et al. “Aberrant DNA Methylation and Gene Expression in Livers of Newborn Mice Transplacentally Exposed to a Hepatocarcinogenic Dose of Inorganic Arsenic.” Toxicology, vol. 236, 2007, pp. 7–15.
Yoshida, T. et al. “Chronic Health Effects in People Exposed to Arsenic via the Drinking Water: Dose–Response Relationships in Review.” Toxicology and Applied Pharmacology, vol. 198, 2004, pp. 243–252.
Yu, Z.M. et al. “What Is the Role of Obesity in the Aetiology of Arsenic-Related Diseases.” Environment International, vol. 66, 2014, pp. 115–123.
Zipes, D.P. et al. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 7th Edn., Elsevier Saunders, Pennsylvania, 2005.