Procurement of Materials:
The seed material, Vignamungo (L.) Hepper was procured from the local market, Tuticorin and was stored in airtight plastic containers at room temperature (25°C±2°C) for further studies.
Experiment 1:
From the plastic containers, 500g seed materials were taken out and used for the following physicochemical parameter analysis.
100 - Seeds Weight (g):
One hundred seeds were taken at random three times and weighed separately. The average weight of 100 seeds was recorded in grams.
Density (Sood et al., 2002): [13]
One-hundred-gram seeds were weighed accurately and transferred to a measuring cylinder. Then 100ml distilled water was added to it. Seed volume was recorded as total volume (ml) – 100ml. Density was recorded as ml per seed.
Hydration Capacity (Sood et al., 2002): [1]3
Seeds weighing 100g were counted and transferred to a measuring cylinder of 500ml capacity and 100ml water was added. The measuring cylinder was covered with aluminium foil and left overnight at room temperature. The next day, seeds were drained, superfluous water was removed with filter paper and swollen seeds were reweighed. Hydration capacity per seed was determined using the following formula.
Hydration capacity = 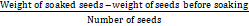
Hydration Index (Sood et al., 2002): [13]
The hydration index was calculated as below
Hydration index = 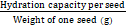
Swelling capacity (Sood et al., 2002): [13]
Seeds weighing 100g were counted; their volume was noted and soaked in 350ml water overnight. The volume of the seed before and after soaking was noted with the help of a graduated cylinder. Swelling capacity per seed was determined using the following formula.
Swelling capacity = 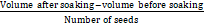
Experiment 2:
Twenty-gram seed material was taken out from the plastic container and was powdered in a Wiley Mill to pass a 60-mesh screen and stored in screw-capped bottles at room temperature and was designated as raw seed powder.
Four lots of seed materials (each lot 20g) were taken out from the plastic containers and put in Petridishes and were exposed to UV-B radiation in the following time intervals.
First lot seed was exposed to UV-B radiation for 15min
Second lot seed was exposed to UV-B radiation for 30min
Third lot seed was exposed to UV-B radiation for 45min
Fourth lot seed was exposed to UV-B radiation for 60min
After the above treatments, the seeds were powered in a Wiley Mill to pass a 60-mesh screen and stored in screw-capped bottles at room temperature and the seed powder obtained by this method was designated as UV-B radiation-exposed seed powder.
Raw as well as treated seed material powders were used for estimating total free phenolics.
Extraction and Estimation of Phenols:
One gram of seed flour was taken in a 100ml flask, to which 50ml of 1% (v/v) HCl in methanol was added. The samples were shaken on a reciprocating shaker for 24h at room temperature. The contents were centrifuged at 10,000 x g for 5min. The supernatant was collected separately and used for further analysis.
One millilitre aliquots of the above extract were transferred into a test tube, to which, 1ml folin-ciocalteu's reagent followed by 2ml of 20% (w/v) Na2Co3 solution was added and the tubes were shaken and placed in a boiling water bath for exactly 1min. The test tubes were cooled under running tap water. The resulting blue solution was diluted to 25ml with distilled water and the absorbance was measured at 650nm with a help of a UV-visible spectrophotometer. The amount of phenols present in the sample was determined from a standard curve prepared with catechol. A blank containing all the reagents minus plant extract was used to adjust the absorbance to zero. The average value of triplicate estimations was expressed as g 100g-1 of the seed flour on dry weight basis.
Experiment 3:
Five lots of seed materials (each lot 20g) were taken out from the containers and put in Pertidishes and were exposed to UV-B radiation in the following time intervals.
The First lot was treated as a control
The second lot was exposed 15min to UV-B radiation
The third lot was exposed 30min to UV-B radiation
The fourth lot was exposed 45min to UV-B radiation
The fifth lot was exposed 60min to UV-B radiation
After that, the seeds were soaked in tap water for 12h and sowed in plastic trays. Thirty-five days after sowing, morphological traits and leaf chlorophyll contents were evaluated.
Experiment 4:
Five lots of seeds were taken out from the containers (each lot 50g) and were soaked in tap water for 12h and then sowed in plastic trays. Twenty-five days after germination (DAG) the plants were exposed to UV-B radiation in the following time intervals:
The plants raised in the first tray were designated as control
The plants raised in the second tray were exposed 15min to UV-B radiation
The plants raised in the third tray were exposed 30min to UV-B radiation
The plants raised in the fourth tray were exposed 45min to UV-B radiation
The plants raised in the fifth tray were exposed 60min to UV-B radiation
Morphological traits and leaf chlorophyll content were recorded after seven days.
Morphological Traits:
Three leaves were randomly harvested from each treatment of UV-B light exposed seeds and UV-B light-exposed plants and their leaf area (cm2) was recorded by using a graph sheet.
Three plants were selected randomly from each treatment and their height (cm), the number of leaves per plant and inter-nodal length (cm) were recorded.
Three plants were randomly taken from the plastic trays as the samples for DM and WC analysis. The weight of fresh matter (FM) of the collected plants was immediately measured using a portable electronic balance (minimum scale: 0.001g). The plants were oven-dried at 130°C for 2h to measure the dry matter (DM) present. The water content of each plant was calculated by subtracting DM from FM (Catchpole & Wheeler, 1992) [16]
One gram of leaf material was taken from the leaf of each treatment and was ground separately with a chilled pestle and mortar in diffuse light with the addition of 20ml of 80% (v/v) cold acetone and the homogenate was centrifuged at 5,000 rpm for 5 minutes. Transferred the supernatant to a 100ml volumetric flask. Again, grounded the residue with 20ml of 80% (v/v) cold acetone, centrifuged and transferred the supernatant to the same volumetric flask. Repeated this procedure until the residue was colourless. The mortar and pestle were thoroughly washed with 80% (v/v) cold acetone and collected the clear washings in the volumetric flask. Made up the volume to 100ml with 80% (v/v) cold acetone. The absorbance of the solution was taken at 645, 663 and 652nm against the solvent (80% cold acetone) blank. Calculated the amount of chlorophyll present in the extract by using the following formula and expressed in mg chlorophyll per g tissue
mg chlorophyll a/g tissue = 12.7 (A 663) – 2.69 (A 645) x 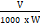
mg chlorophyll b/g tissue = 22.9 (A 645) – 4.68 (A 663) x 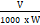
mg total chlorophyll/g tissue = 20.2 (A 645) + 8.02 (A 663) x 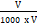
Where,
A = Absorbance at a specific wavelength.
V = Final volume of chlorophyll extract in 80% acetone.
W = Fresh weight of tissue extract.
Statistical Analysis:
100-seed weight, density, swelling capacity, hydration capacity, hydration index, total free phenolics, plants height (cm), leaf area (cm2), inter-nodal length, DM, WC and leaf chlorophyll were estimated on triplicate determinations. Estimates of mean and standard error for the above-stated parameters were calculated with help of a calculator.


