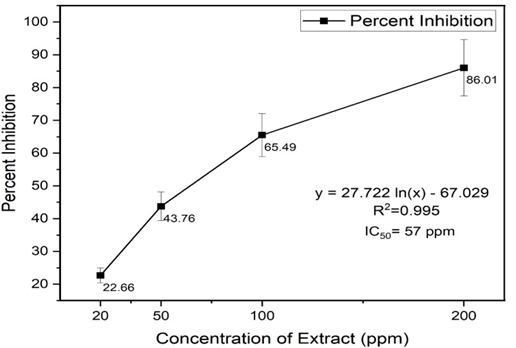
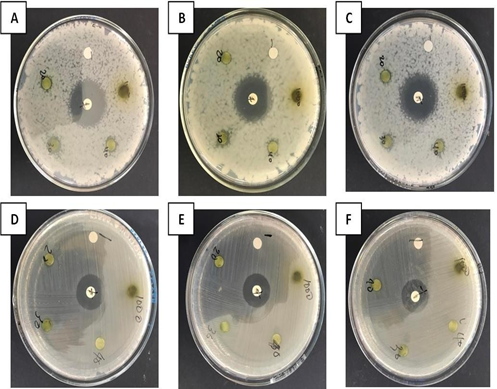
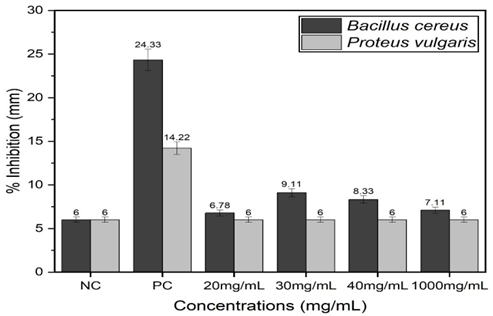
Iwu, M. W., A. R. Duncan, and C. O. Okunji. "New Antimicrobials of Plant Origin." ASHS Press, 1999, pp. 457-462.
Govindarajulu, B., et al. "Mosquito Larvicidal Efficacy of the Leaf Extracts of Annona reticulata Against Aedes aegypti." International Journal of Current Microbiology and Applied Sciences, vol. 4, 2015, pp. 132-140.
Rupa, D., Y. C. Sulistyaningsih, D. Dorly, and D. Ratnadewi. "Identification of Secretory Structure, Histochemistry and Phytochemical Compounds of Medicinal Plant Hyptis capitata Jacq." Biotropia, vol. 24, no. 2, 2017, pp. 94-103. doi: 10.11598/btb.2017.24.2.499.
Kusuma, I. W., et al. "Biological Activities and Phytochemicals of Hyptis capitata Grown in East Kalimantan, Indonesia." Journal of Applied Biology & Biotechnology, vol. 8, no. 2, 2020, pp. 58-64. doi: 10.7324/jabb.2020.80210.
DeFilipps, R. A., S. L. Maina, and J. Crepin. Medicinal Plants of the Guianas (Guyana, Surinam, French Guiana). Smithsonian Institution Press, 2003.
Ahmad, F. B., and D. K. Holdsworth. "Medicinal Plants of Sabah, East Malaysia – Part I." Pharmaceutical Biology, vol. 41, no. 5, 2008, pp. 340-346. doi: 10.1076/phbi.41.5.340.15940.
Biswas, A., M. A. B. Miah, M. Roy, and S. Bhadra. "Inherited Folk Pharmaceutical Knowledge of Tribal People in the Chittagong Hill Tracts Bangladesh." Indian Journal of Traditional Knowledge, vol. 9, 2010, pp. 77-89.
Gailea, R., A. A. Bratawinata, R. Pitopang, and I. W. Kusuma. "The Use of Various Plant Types as Medicines by Local Community in the Enclave of the Lore-Lindu National Park of Central Sulawesi, Indonesia." Global Journal of Research on Medicinal Plants & Indigenous Medicine, vol. 5, no. 1, 2016, pp. 29-40.
Dapar, M. L. G. Ethnobotany of the Mountain Regions of Southeast Asia. Springer, 2020.
"Labor Market Intelligence Report." Technical Education and Skills Development Authority, issue 3, 2020.
Dapar, M. L. G., et al. "Ethnomedicinal Plants Used for the Treatment of Cuts and Wounds by the Agusan Manobo of Sibagat, Agusan del Sur, Philippines." Ethnobotany Research and Applications, vol. 19, 2020. doi: 10.32859/era.19.31.1-18.
Lañojan, R., et al. "Ethnomedicinal Resources of the Indigenous People’s (IP) Groups in the SOCSARGEN Region." Journal of Health Research and Society, vol. 1, 2018. doi: 10.34002/jhrs.v1i0.8.
Olowa, L., and C. Demayo. "Ethnobotanical Uses of Medicinal Plants Among the Muslim Maranaos in Iligan City, Mindanao, Philippines." Advances in Environmental Biology, vol. 9, no. 27, 2015, pp. 204-215.
Cordero, C., and G. J. D. Alejandro. "Medicinal Plants Used by the Indigenous Ati Tribe in Tobias Fornier, Antique, Philippines." Biodiversitas: Journal of Biological Diversity, vol. 22, no. 2, 2021. doi: 10.13057/biodiv/d220203.
Tantengco, O. A. G., et al. "Ethnobotanical Survey of Medicinal Plants Used by Ayta Communities in Dinalupihan, Bataan, Philippines." Pharmacognosy Journal, vol. 10, no. 5, 2018, pp. 859-870. doi: 10.5530/pj.2018.5.145.
Arquion, R. D., et al. "Ethnobotanical Study of Indigenous Plants Used by Local People of Agusan del Sur, Philippines." Asia Pacific Higher Education Research Journal, vol. 2, no. 2, 2015. doi: 10.56278/apherj.v2i2.102.
Campilan, J. R., et al. "Quantitative Ethnobotanical Study, Phytochemical Screening and Antibacterial Assay of Ethnomedicinal Plants of T’boli in Lemsnolon, Tboli, South Cotabato." International Journal of Pharmacology, Phytochemistry and Ethnomedicine, vol. 13, 2019, pp. 45-61. doi: 10.18052/www.scipress.com/IJPPE.13.45.
Tafurt García, G., L. Jiménez Vidal, and A. Calvo Salamanca. "Antioxidant Capacity and Total Phenol Content of Hyptis spp., P. heptaphyllum, T. panamensis, T. rhoifolia, and Ocotea sp." Revista Colombiana de Química, vol. 44, no. 2, 2016, pp. 28-33. doi: 10.15446/rev.colomb.quim.v44n2.55217.
Falcão, D. Q., et al. "Triterpenos de Hyptis fasciculata Benth." Revista Brasileira de Farmacognosia, vol. 13, 2003, pp. 81-83. doi: 10.1590/s0102-695x2003000300030.
Shan, B., et al. "Antioxidant Capacity of 26 Spice Extracts and Characterization of Their Phenolic Constituents." Journal of Agricultural and Food Chemistry, vol. 53, no. 20, 2005, pp. 7749-7759. doi: 10.1021/jf051513y.
McNeil, M., P. Facey, and R. Porter. "Essential Oils from the Hyptis Genus- A Review (1909-2009)." Natural Product Communications, vol. 6, no. 11, 2011. doi: 10.1177/1934578x1100601149.
Bezerra, J. W. A., et al. "Chemical Composition and Toxicological Evaluation of Hyptis suaveolens (L.) Poiteau (Lamiaceae) in Drosophila melanogaster and Artemia salina." South African Journal of Botany, vol. 113, 2017, pp. 437-442. doi: 10.1016/j.sajb.2017.10.003.
Harborne, J. B. Phytochemical Methods, 3rd ed., Chapman and Hall, 1973.
Alam, M. N., N. J. Bristi, and M. Rafiquzzaman. "Review on in Vivo and in Vitro Methods Evaluation of Antioxidant Activity." Saudi Pharmaceutical Journal, vol. 21, no. 2, 2013, pp. 143-152. doi: 10.1016/j.jsps.2012.05.002.
Pisoschi, A. M., and G. P. Negulescu. "Methods for Total Antioxidant Activity Determination: A Review." Biochemistry & Analytical Biochemistry, vol. 01, no. 01, 2012. doi: 10.4172/2161-1009.1000106.
Oyeyinka, B. O., and A. J. Afolayan. "Comparative and Correlational Evaluation of the Phytochemical Constituents and Antioxidant Activity of Musa sinensis L. and Musa paradisiaca L. Fruit Compartments (Musaceae)." The Scientific World Journal, vol. 2020, 2020, pp. 1-12. doi: 10.1155/2020/4503824.
Waterhouse, A. L. "Determination of Total Phenolics." Current Protocols in Food Analytical Chemistry, 2003. doi: https://doi.org/10.1002/0471142913.fai0101s06.
Biemer, J. "Antimicrobial Susceptibility Testing by the Kirby-Bauer Disc Diffusion Method." Annals of Clinical Laboratory Science, vol. 3, no. 2, 1973.
M. C., D. "Proximate, Phytochemical and Nutrient Compositions of Some Fruits, Seeds and Leaves of Some Plant Species at Umudike, Nigeria." ARPN Journal of Agricultural and Biological Science, vol. 5, no. 1, 2010.
Anyanwu, C. U., and G. C. Nwosu. "Assessment of the Antimicrobial Activity of Aqueous and Ethanolic Extracts of Piper guineense Leaves." Journal of Medicinal Plants Research, vol. 8, no. 10, 2014, pp. 436-440. doi: 10.5897/jmpr12.976.
Heinrich, M., J. Mah, and V. Amirkia. "Alkaloids Used as Medicines: Structural Phytochemistry Meets Biodiversity—An Update and Forward Look." Molecules, vol. 26, no. 7, 2021, p. 1836. https://doi.org/10.3390/molecules26071836.
Hannan, J. M. A., et al. "Evaluation of Phytochemical Screening and Antimicrobial Activities of Ethanolic Extracts of Leaves and Bark of Ardisia Colorata." International Journal of Pharmaceutical and Life Sciences, vol. 2, no. 4, 2013, pp. 158-164. https://doi.org/10.3329/ijpls.v2i4.17115.
Auwal, M. S., et al. "Preliminary Phytochemical and Elemental Analysis of Aqueous and Fractionated Pod Extracts of Acacia Nilotica (Thorn Mimosa)." Veterinary Research Forum, vol. 5, no. 2, 2014, pp. 95-100.
Kar, A. Pharmacognosy and Pharmacobiotechnology. 2nd ed., New Age International, 2007, p. 898.
Moghimipour, E., and S. Handali. "Saponin: Properties, Methods of Evaluation and Applications." Annual Research & Review in Biology, vol. 5, no. 3, 2015, pp. 207-220. https://doi.org/10.9734/arrb/2015/1167
Tiwari, P., et al. "Phytochemical Screening and Extraction: A Review." Internationale Pharmaceutica Scientia, vol. 1, no. 1, 2011.
Su, Y., et al. "Antioxidant Activity of Flavonoids Isolated from Scutellaria Rehderiana." Journal of the American Oil Chemists' Society, vol. 77, no. 8, 2000, pp. 807-812. https://doi.org/10.1007/s11746-000-0129-y.
Chien, S. C., et al. "Naturally Occurring Anthraquinones: Chemistry and Therapeutic Potential in Autoimmune Diabetes." Evid Based Complement Alternat Med., vol. 2015, p. 357357, 2015. https://doi.org/10.1155/2015/357357.
Ghasemi Pirbalouti, A., et al. "Antioxidant Activity, Total Phenolic and Flavonoid Contents of Some Medicinal and Aromatic Plants Used as Herbal Teas and Condiments in Iran." Journal of Medicinal Food, vol. 17, no. 10, Oct. 2014, pp. 1151-1157. https://doi.org/10.1089/jmf.2013.0057.
Chikara, S., et al. "Oxidative Stress and Dietary Phytochemicals: Role in Cancer Chemoprevention and Treatment." Cancer Letters, vol. 413, 28 Jan. 2018, pp. 122-134. https://doi.org/10.1016/j.canlet.2017.11.002.
Fazeli-Nasab, B., et al. "Investigation of Antimicrobial Activity of Medicinal Plant Extracts on Bacillus Cereus Isolated from Soil." Gene, Cell and Tissue, vol. 9, no. 1, 2021. https://doi.org/10.5812/gct.115133.
Samy, R. P., and S. Ignacimuthu. "Antibacterial Activity of Some Folklore Medicinal Plants Used by Tribals in Western Ghats of India." Journal of Ethnopharmacology, vol. 69, no. 1, Jan. 2000, pp. 63-71. https://doi.org/10.1016/s0378-8741(98)00156-1.
Gutierrez, R. M., et al. "Antibacterial Potential of Some Medicinal Plants of the Cordillera Region, Philippines." Indian Journal of Traditional Knowledge, vol. 12, no. 4, 2013, pp. 630-637.
Ameri, A., et al. "Medicinal Plants Contain Mucilage Used in Traditional Persian Medicine (TPM)." Pharmaceutical Biology, vol. 53, no. 4, 2014, pp. 615-623. https://doi.org/10.3109/13880209.2014.928330.
Norajit, K., et al. "Antibacterial Effect of Five Zingiberaceae Essential Oils." Molecules, vol. 12, no. 8, 23 Aug. 2007, pp. 2047-2060. https://doi.org/10.3390/12082047.
Vaara, M. "Antibiotic-Supersusceptible Mutants of Escherichia Coli and Salmonella Typhimurium." Antimicrobial Agents and Chemotherapy, vol. 37, no. 11, Nov. 1993, pp. 2255-2260. https://doi.org/10.1128/AAC.37.11.2255.
Nikaido, H. "Multidrug Efflux Pumps of Gram-Negative Bacteria." Journal of Bacteriology, vol. 178, no. 20, Oct. 1996, pp. 5853-5859. https://doi.org/10.1128/jb.178.20.5853-5859.1996.
Piddock, L. J. "Clinically Relevant Chromosomally Encoded Multidrug Resistance Efflux Pumps in Bacteria." Clinical Microbiology Reviews, vol. 19, no. 2, Apr. 2006, pp. 382-402. https://doi.org/10.1128/CMR.19.2.382-402.2006.