Detection of spa Gene in S. aureus
In the current investigation, the existence of the (spa) gene in S. aureus isolates from UTI patients was detected using the PCR technique. Each gene is displayed by a single band in the relevant section of the DNA ladder, which represents the gene's location (1500-100bp). All S. aureus isolates (n=55) were amplificated to spa gene at (1400-260 bp) with a positive reaction of 55 (100%)
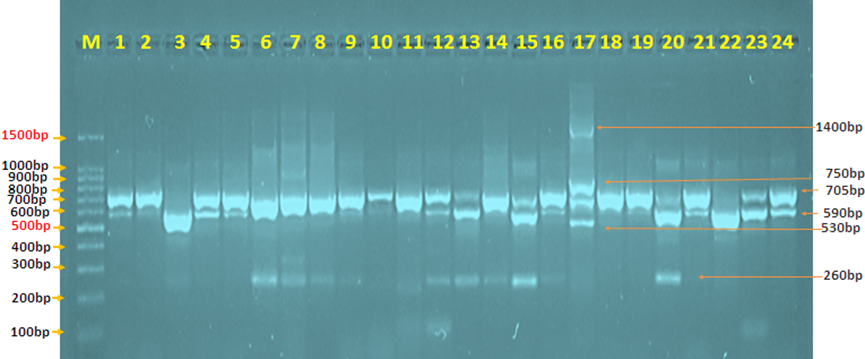
Figure 4.15: 1 Agarose gel electrophoresis picture of the PCR product analysis of the Staphylococcus aureus protein a (spa) gene in Staphylococcus aureus isolates from urinary tract infections. Where the marker ladder (1500-100bp) and lane (1-24) revealed some positive spa gene at (1400-260 bp) PCR product size.
Detection of tst-1 Gene in S. aureus
The occurrence of the (tst-1) gene in S. aureus isolated from UTI patients was discovered in this study utilizing the PCR method. Each gene is represented by a single band in the DNA ladder's corresponding region (1500-100bp). All S. aureus isolates (n=55) were amplificated to tst-1 gene at (538bp) with a positive reaction of 11 (20%)

Figure 4.15: 1 Agarose gel electrophoresis picture of the PCR product analysis of the toxic shock syndrome toxin-1 (tst-1) gene in Staphylococcus aureus isolates from urinary tract infections. Where lane (1-20) exhibited some positive tst-1 gene at (538bp) PCR product size, and marker ladder (1500-100bp)
The polymorphism in the gene that codes for a protein. People are typing in a spa. In addition to the five IgG binding sites (A, B, C, D, and E), Protein A features a cell wall attachment domain at the C-terminus. The Fc-binding domain of the protein spa is encoded by one region, whereas the X region is encoded by the other [9].
The Spa type method exploits tandem repeats and sequence diversity in protein region X. Gene. Several investigations have shown varied spa gene patterns across S. aureus strains obtained from patients in various parts of the world [10].
In the current investigation, the genotypic identification rate of spa gene in UTIs samples was 24(100%) with different number of repeat polymorphism site, especially in UTI.17 with four sites, band size (1400-260 bp) and IgG binding sites, the result of the current study seems to be in agreement with (Goudarzi, Mehdi et al., 2018) [11]. but the results are not comparable with (Goudarzi, Mehdi et al., 2019) [12] they mentioned the percentage is (25.3%). These differences may be due to the sources and number of the clinical samples used, and the sensitivity of different techniques used or my be due to geographical dependency.
S. aureus superantigens (SAgs) are a unique group of non-glycosylated low-molecular-weight exoproteins that are very resistant to heat, acids, proteolysis, and desiccation. SAgs are also very resistant to being dehydrated [13]. This toxin family can cause excessive T-cell activation and cytokine production, affecting immune system function throughout the body.
Because of their biological toxicity, SAgs are important contributors to life-threatening illnesses. When expressed in a susceptible host, the toxic shock syndrome toxin-1 (TSST-1) gene codes for a member of the SAgs family of toxins that can induce staphylococcal toxic shock syndrome (TSS) [13].
In the current work, all S. aureus isolates were amplificated to tst-1 gene at (538bp) with a positive reaction of 4 (20%),it is nearly similar to (Alni, Reza Hakimi et al.,2018), (Wang, Min et al.,2017) [14-15] they mentioned the percentage is (22%),(18%) correspondingly. The tst-1 gene ratio was slightly higher in other studies, with the tst-1 gene being found in (26.31%) and (44.4%) tst-1-positive. [11, 16]




