The research study was conducted in Kebbi State, and the different soil type samples were collected from Birnin Kebbi Local Government Area, the State Capital of Kebbi State of the Northwestern part of Nigeria. The State has a total area of 36,800km2; it lies between latitude 12.450N in the north and 4.20E to the east. The state has three seasons mainly, dry, rainy, and harmattan seasons. The temperature of the State usually ranges from a maximum of 42.70C to a minimum of 24.90C. The major types of soil in Kebbi State just like many other regions are sandy, clay, and loamy soil.[15].
2.1 Collection of Samples (Collection of crude oil/ Soil Samples)
Nigerian crude oil (Bonny light crude oil) was collected from Kaduna Refinery Petrochemical Company (KRPC), Kaduna Nigeria. About 500-600 grams of different soil types sandy, loamy, and clay soil were collected within Birnin Kebbi Local Government Area of Kebbi State. Different concentrations of sterilized crude oil (5ml, 10ml, and 15ml) were used to artificially contaminate a fixed amount of Sandy soil collected using the probe into three conical flasks which were labeled A1, A2, and A3 for 5ml, 10ml, and 15ml crude oil respectively. One conical flask for control containing Sandy soil was left uncontaminated and regarded as control, the flask was labeled AC. The same procedures were performed for other Clay and Loamy soils.
The samples were placed in a clean plastic container and labeled A, B, and C for sandy, clay, and loamy respectively. The samples were then transported to the microbiology laboratory, biological science department of Kebbi State University of Science and Technology, Aliero, for processing.
2.2 Selection of hydrocarbon tolerant strains
To select the hydrocarbon utilizing or hydrocarbon tolerating bacteria, different concentrations of sterilized crude oil (5ml, 10ml, and 15ml) were used to artificially contaminate a fixed amount of Sandy soil in three conical flasks which were labeled A1, A2, and A3 for 5ml, 10ml, and 15ml crude oil respectively. One conical flask for control containing Sandy soil was left uncontaminated and regarded as control, the flask was labeled AC. The same procedures were performed for other Clay and Loamy soils.
The labeling followed the order given below:
A1 A2 A3 AC - Sandy Soil
B1 B2 B3 BC - Clay Soil
C1 C2 C3 CC - Loamy Soil
All contaminated and control flasks were incubated at room temperature for seven days to select the crude oil tolerant strains.
2.3 Media Preparation
All media used in this research work was prepared according to the manufacturer’s instructions.
2.4 Bacteriological Analysis
2.5 Isolation, Enumeration, and characterization of Crude oil Degrading Bacteria from different soil type samples contaminated with crude oil.
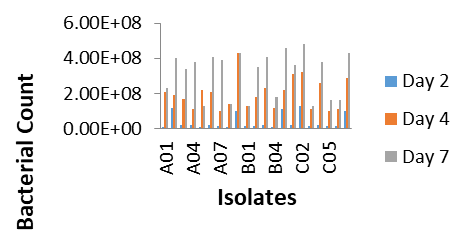
After the period of incubation, Ringer’s solution was prepared, 9ml was aliquot into test tubes, the Ringer’s solution was then autoclaved and allowed to cooled, 1g of each soil treatment was serially diluted into the cooled ringer solution, dilution factor 6 7 and 8 were inoculated on a crude oil base media and then incubate at 37 0C for 24hrs.After the incubation period colonies were counted, sub cultured on the same media, and incubated at the same temperature and period for further characterization and identification. The cultural and morphological characterization of the isolates was carried out using the standard microbiological method.
2.5.1Gram staining
A drop of normal saline was placed on a clean slide; a sterile wire loop was used to pick a colony from the Petri-dish which was emulsified on the slide to make a smear. The smear was heat fixed by passing over the flame. It was then covered with crystal violet stain for 1 minute and washed off with distilled water. This was followed by covering the smear with Lugol's iodine for 1 minute; it was washed off with distilled water and decolorized rapidly with acetone-alcohol for 30 seconds; then washed immediately with distilled water. The smear was flowed with safranin and left for 1 minute after which it was washed with distilled water and allowed to dry[16]. An oil immersion was placed on the surface of the smear and the slide was viewed with a microscope using x 100 objectives.
2.6 Biochemical Characterization of Bacterial Isolates
Coagulase test
Two drops of physiological saline were placed on a clean, grease-free slide. A colony of the test organism (previously checked by Gram’s Staining) was emulsified on the drop of the physiological saline. Loopful of plasma was added to the suspension and mixed gently. Clumping of organisms within 10 seconds indicated a positive coagulase test while the absence of clumping within 10 seconds indicated a negative coagulase test.
Catalase Test
A catalase test was done by placing a drop of 3% hydrogen peroxide on a clean, grease-free slide. With sterile inoculating wire, a colony of the organism was picked and placed in the 3% hydrogen peroxide. The presence of bubbles indicated catalase positive while the absence of bubbles indicated a catalase-negative result[16].
Oxidase Test
A piece of filter paper was placed in a clean Petri dish and 3 drops of freshly prepared oxidase reagent were added. Using a glass rod, a colony of the test organism was removed and smeared on the filter paper containing the oxidase reagent. The development of a blue-purple color within 10 seconds indicated a positive result while the absence of blue-purple color within 10 seconds indicated a negative result[16].
Urease Activity
This test is used to detect the organism's ability to produce enzyme urease that hydrolyses urea into ammonia and carbon dioxide. When the strain is urease-producing, the enzyme will break down the urea by hydrolysis to give ammonia and carbon dioxide. With the release of ammonia, the pale yellow of urea changes to pink-red which signifies positive for urease. Colony from the stock culture was subcultured into nutrient agar to obtain a fresh culture. Heavy inoculums were fetched from the nutrient agar using a sterile wire loop and streaked on the slant surface of the urea medium. It was incubated for 24hrs at 370c. The development of a pink/red signifies urease positive; if color remains unchanged (yellow/orange) it signifies negative[16].
Citrate Test
This test is one of the several techniques used to assist in the identification of some groups of bacteria. The test is based on the ability of an organism to use citrate as its source of carbon. Simon citrate agar was inoculated with the isolate and incubation was done at 370C for 48hrs. The presence of a bright blue color indicates citrate positive i.e. the organism utilizes citrate as its source of carbon while when there is no change in the color of the medium, it indicates citrate negative[16].
Nitrate Reduction Test
About 0.5 mL of the sterile nitrate broth was inoculated with the test organism and incubated at 370C for 4hours. 1 drop of sulphanilic acid reagent and one drop of alpha-naphthylamine reagent were added. The test tube was shaken and observed for the presence of red color, which indicates a positive result. The absence of red color indicates a negative result [16].
Indole Test
This test has used the determination of the ability of bacteria to produce Indole from tryptophan. Indole production is detected by Kovac's reagent which contains 4–dimethylaminobenzaldehyde. The reaction of the reagent with indole produces a red-colored compound. The isolate was grown for 48hrs in a test tube containing 5ml peptone water; 0.5ml of Kovac's reagent was added and shaken gently. The presence of a red or pink layer signifies the presence of indole which also indicates positive for indole; for indole negative, the color remains yellow[16].
VIII. Methyl Red-Voges Proskauer (MRVP) Test
This test is used in differentiating bacteria that ferment glucose with the production of acetyl methyl carbonyl (acetone). The media contains peptone salt and glucose. Five (5) milliliters of methyl red- Voges-Proskauer broth was inoculated with the test organism and incubated at 35°C for 48h. After the period of incubation, one (1) milliliter of the broth was transferred into a test tube and five (5) drops of methyl red reagent were added. The red color on the addition of the indicator signified a positive methyl red reaction while the yellow color signified a negative test[17].
Triple Sugar Iron Agar Test
This medium contains three sugar namely glucose, sucrose, and lactose. Some organism can ferment all three sugars present and produces acid which changes the color of the indicator from red to yellow. The inoculum was streaked on the surface of the TSI agar slant and stabbed using a sterile wire loop and incubated at 37°C for 24h. After the incubation period, the tubes were observed for sugar fermentation, hydrogen sulfide, and gas production. The gas formation was determined by the appearance of one or several bubbles in the bottom. The formation of hydrogen sulfide was determined by the blackening of the whole bottom or a streak or ring of blackening at the slant bottom junction. The bottom becoming yellow indicated glucose fermentation. If no other sugar was fermented, the slant was red while the bottom was yellow[17].
Mannitol Fermentation Test.
The purpose of the test was to see if the isolates can ferment mannitol as a carbon source. Inoculums from a pure culture were transferred aseptically using a sterile wire loop to a sterile tube of phenol red mannitol broth. The inoculated tubes were then incubated at 35 – 37 oC for 24 hours and the results were determined. The positive test consists of a color change from red to yellow, indicating a pH change to acidic, while the negative test was indicated by hot pink color.
XI. Motility Test
The stabbing technique was used to perform this test. Test tubes containing sterilized sulfide, indole, and motility (SIM) agar were prepared. The sterilized inoculating needle was used to pick up isolates from their pure cultures. Each test tube was stabbed with the needle rubbed with each isolate in the middle. The test tubes were then incubated at 37°C for 24h after which the tubes were observed for the motility. A motile organism mostly grew away from the point where the medium was stabbed[17].
XII. Spore Staining
A smear of the bacterial colony was prepared using a sterile wire loop. It was heat fixed by passing it over a Bunsen flame. A drop of 5% malachite green was added. It was then washed off with distilled water and 0.5% safranin was added to the smear for 30 seconds, washed, and air-dried. The slide was viewed using the oil immersion objective (x100). The spore stained green under the microscope [17] while the vegetative cell appeared pink.
2.7 STATISTICAL ANALYSIS
Data obtained from the study were analyzed using IBM SPSS version 23 to determine the counts. Descriptive statistical tests were used to describe the data.